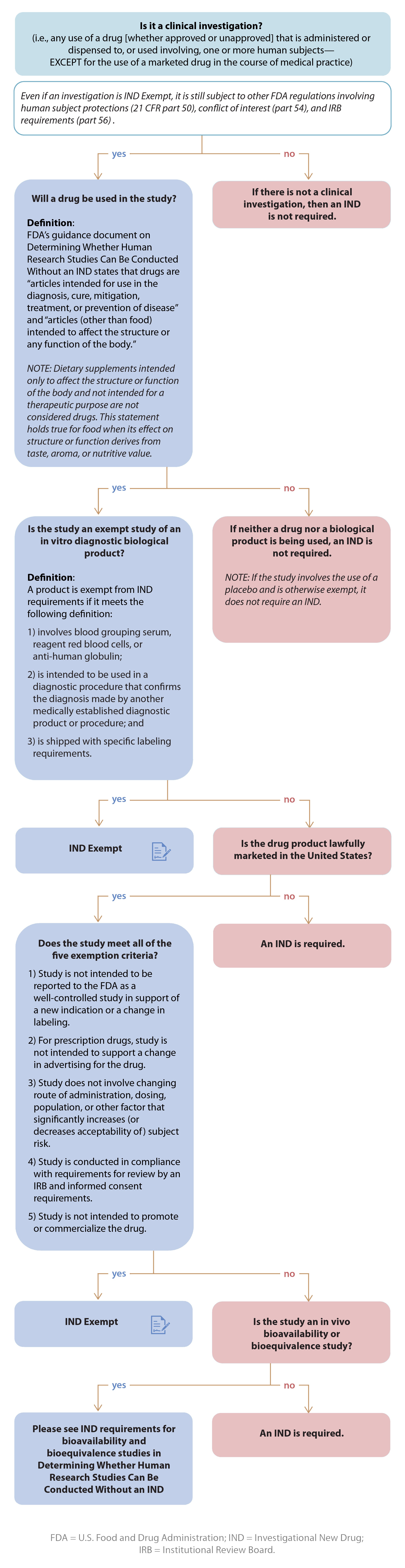
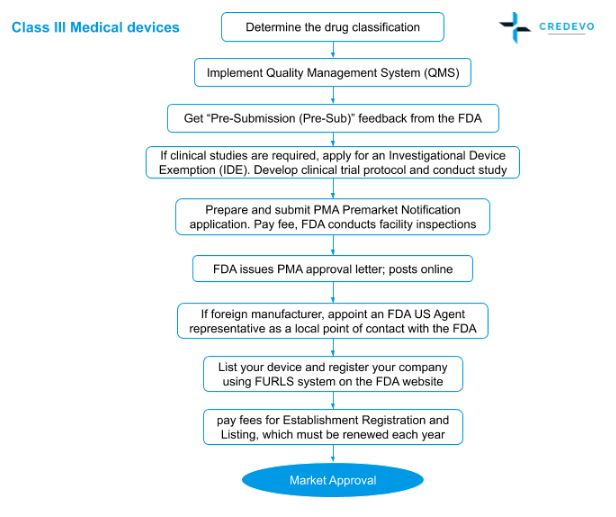
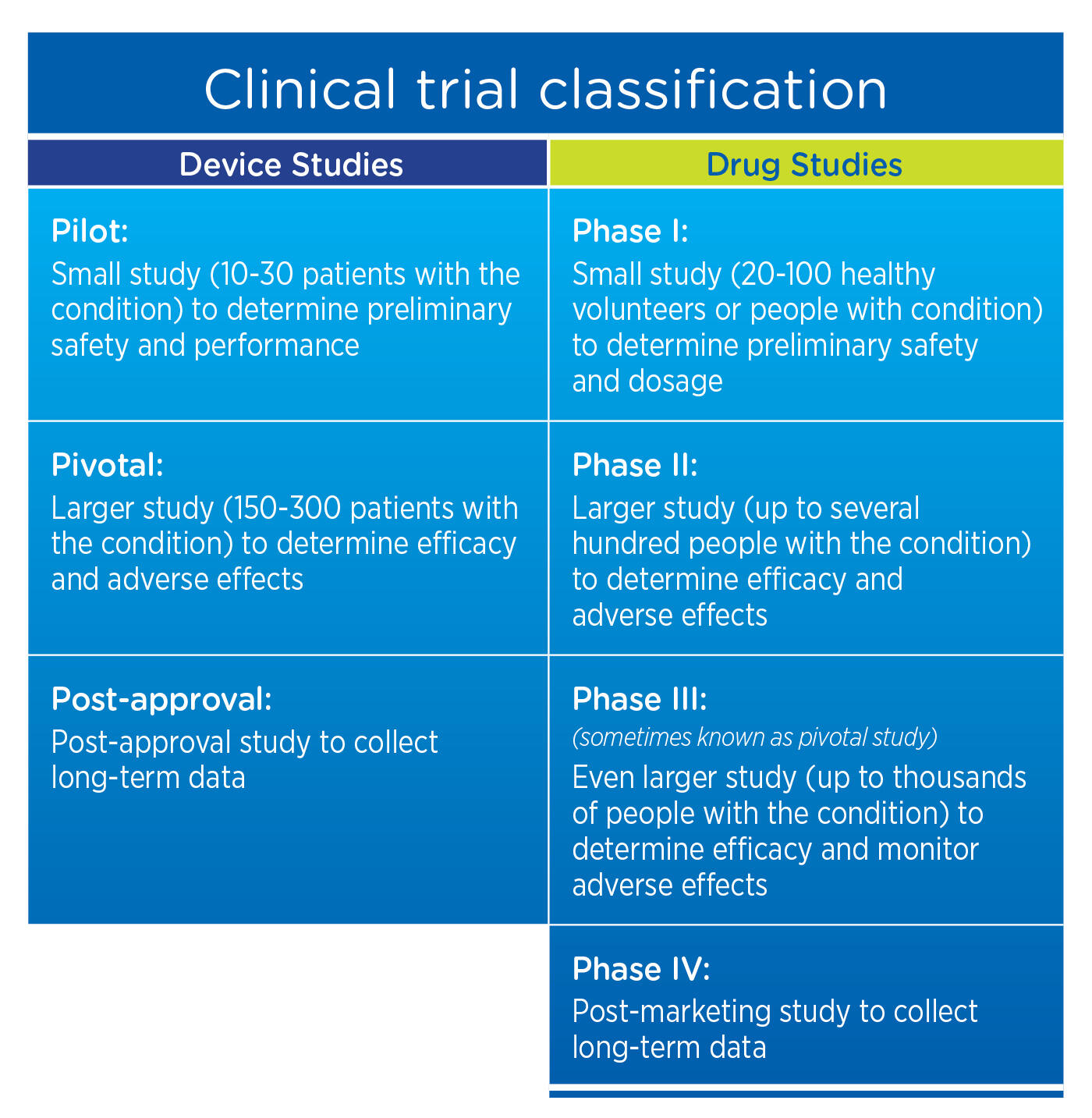
IDE DECISION WORKSHEET For Investigator-Initiated Clinical Investigations Does Your Study Require an IDE Submittal to the FDA? N

Updated FDA Guidance Helps Device Study Sponsors Better Anticipate Coverage for Investigational Devices | Advisories | Arnold & Porter
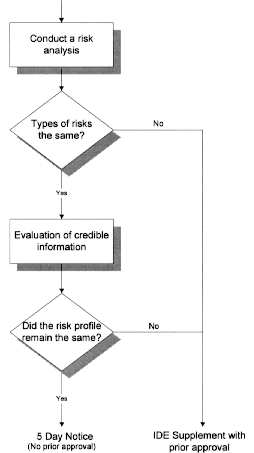
Changes or Modifications During the Conduct of a Clinical Investigation; Final Guidance for Industry and CDRH Staff | FDA

FDA Regulation of Neurological and Physical Medicine Devices: Access to Safe and Effective Neurotechnologies for All Americans - ScienceDirect