Sustained Effects of Different Exercise Modalities on Physical and Mental Health in Patients With Coronary Artery Disease: A Randomized Clinical Trial - Canadian Journal of Cardiology

Adjuvant melatonin for the prevention of recurrence and mortality following lung cancer resection (AMPLCaRe): A randomized placebo controlled clinical trial - eClinicalMedicine

Analysis of pooled clinical trial data: incidence of malignancy over... | Download Scientific Diagram
Are cancer patients better off if they participate in clinical trials? A mixed methods study | BMC Cancer | Full Text

Radiology workflow for RECIST assessment in clinical trials: Can we reconcile time-efficiency and quality? - European Journal of Radiology

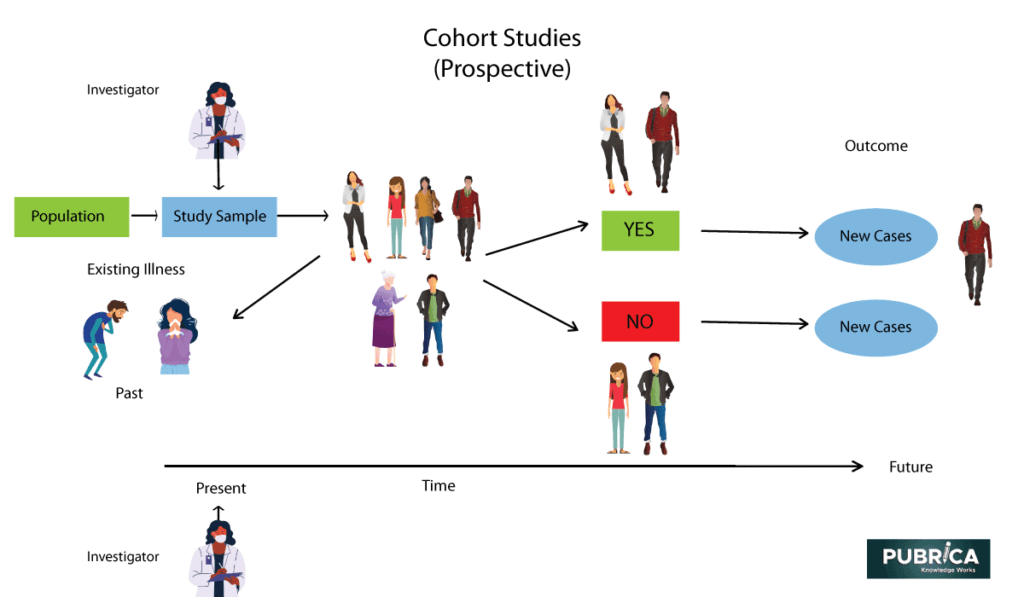
Follow-up time for each cohort/clinical trial included in the MULTI... | Download Scientific Diagram

Optimal design of clinical trials with computer simulation based on results of earlier trials, illustrated with a lipodystrophy trial in HIV patients - ScienceDirect

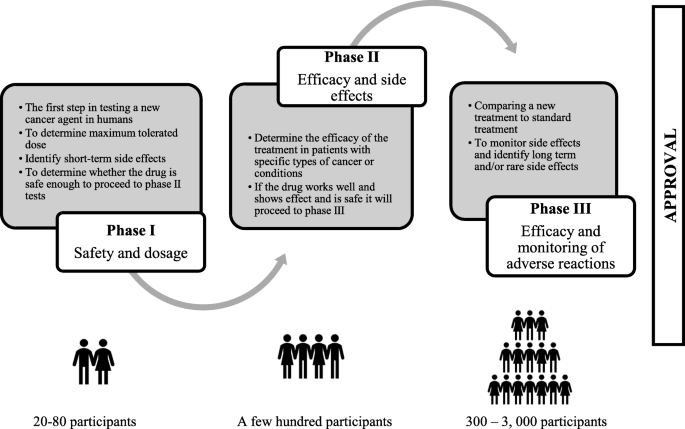
EPIC: an evaluation of the psychological impact of early-phase clinical trials in cancer patients - ESMO Open

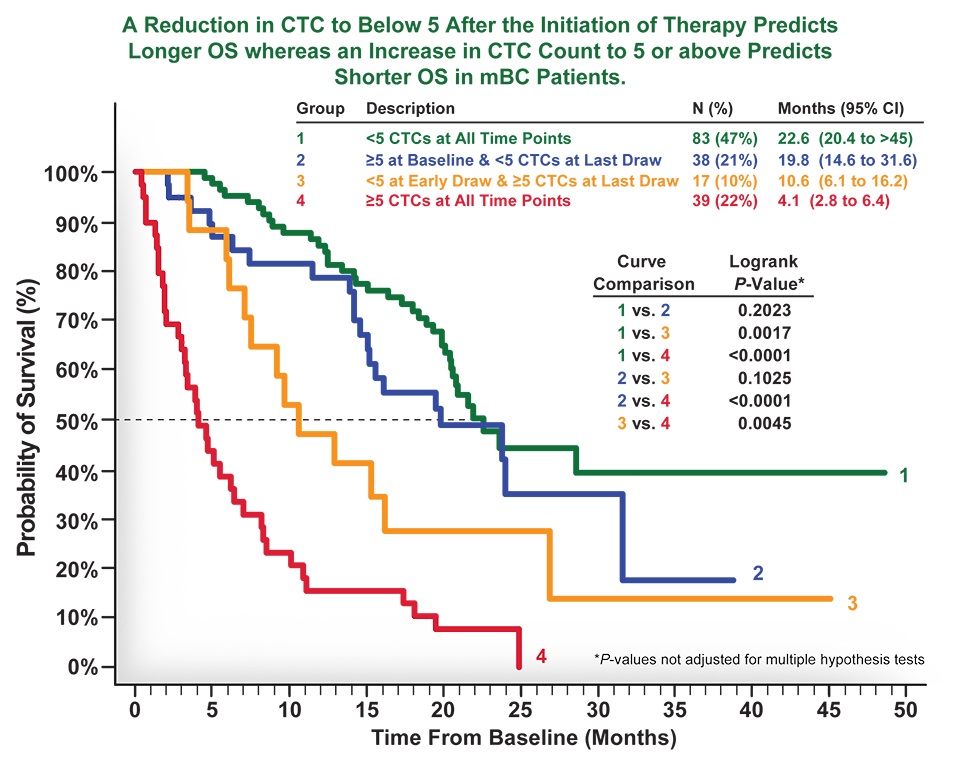
An Application of the Patient Rule-Induction Method to Detect Clinically Meaningful Subgroups from Failed Phase III Clinical Trials

Timeline (in weeks) of the clinical trial illustrating the intervention... | Download Scientific Diagram

Protocol of the Definition for the Assessment of Time-to-event Endpoints in CANcer trials (DATECAN) project: Formal consensus method for the development of guidelines for standardised time-to-event endpoints' definitions in cancer clinical trials -

DAHLIA project overview including highlights of each study and time... | Download Scientific Diagram

Schematic of data collection and timing. The initial therapy period for... | Download Scientific Diagram

Incorporating Site-less Clinical Trials Into Drug Development: A Framework for Action - Clinical Therapeutics