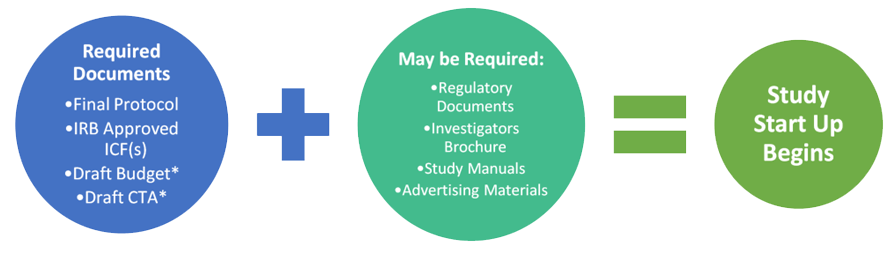
What you Need and When – The Key Documents in the Drug Lifecycle - Trilogy Writing & Consulting GmbH

What you Need and When – The Key Documents in the Drug Lifecycle - Trilogy Writing & Consulting GmbH
![PDF] Informed consent in clinical research: Consensus recommendations for reform identified by an expert interview panel | Semantic Scholar PDF] Informed consent in clinical research: Consensus recommendations for reform identified by an expert interview panel | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/3f6b229a2937974f4f37094037fcb258b6a8d47d/3-Figure1-1.png)
PDF] Informed consent in clinical research: Consensus recommendations for reform identified by an expert interview panel | Semantic Scholar
Informed consent in oncology clinical trials: A Brown University Oncology Research Group prospective cross-sectional pilot study | PLOS ONE

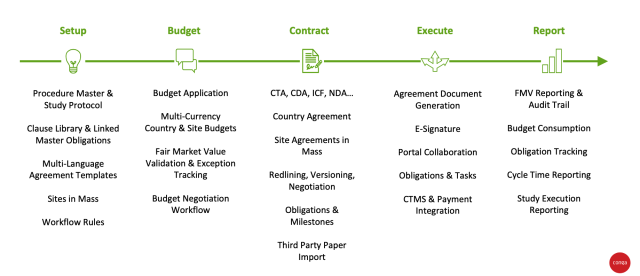
PDF) Project Management of Randomized Clinical Trials: A Narrative Review | Hamidreza Goodarzynejad - Academia.edu

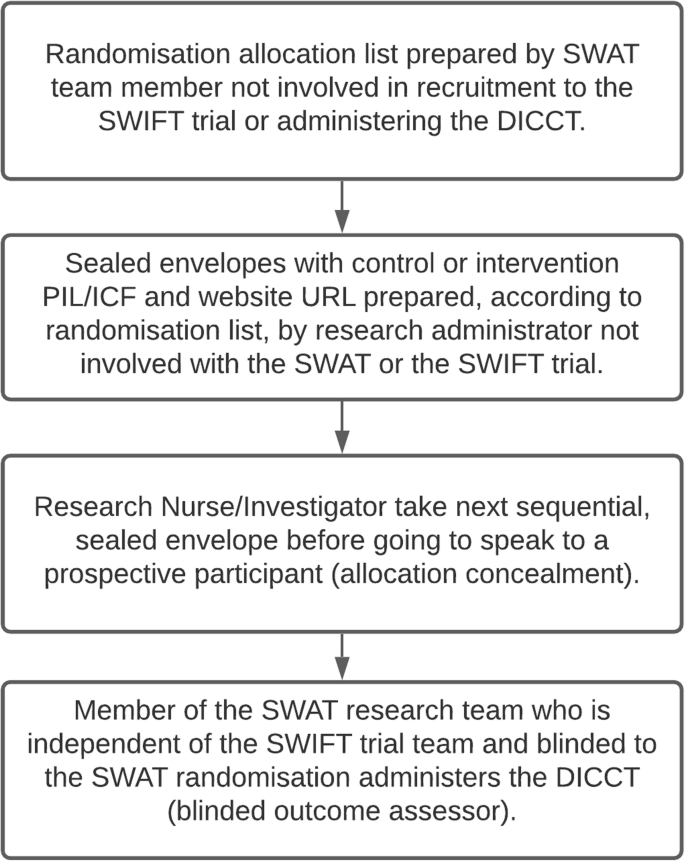
Considerations for obtaining informed consent. ICF, Informed consent... | Download Scientific Diagram